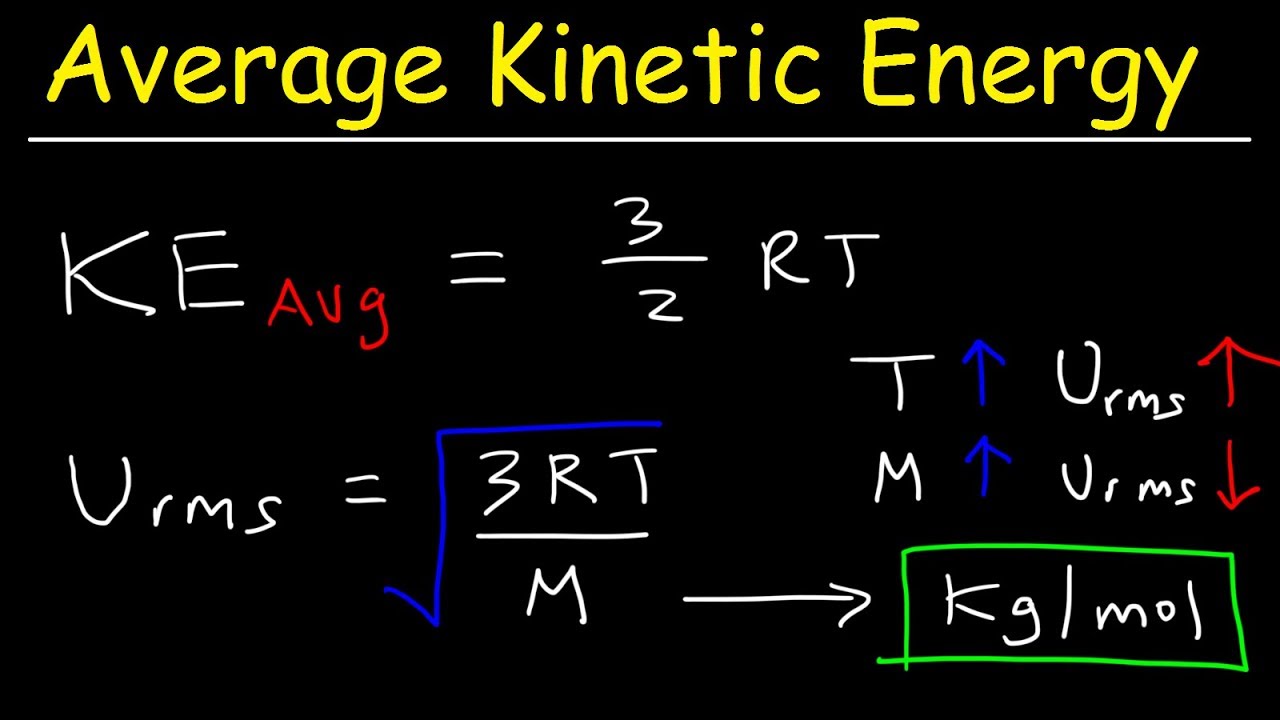
 This chemistry video tutorial explains how to calculate the average kinetic energy of a gas and the root mean square velocity as well. It contains plenty of examples and practice problems on this topic. This video contains all of the equations and formulas needed to solve the problems presented in this tutorial. The average kinetic energy of a collection of gas particles is directly proportional to the kelvin temperature. The root mean square velocity increases with increasing temperature but decreases as the molar mass of a gas increases.
This chemistry video tutorial explains how to calculate the average kinetic energy of a gas and the root mean square velocity as well. It contains plenty of examples and practice problems on this topic. This video contains all of the equations and formulas needed to solve the problems presented in this tutorial. The average kinetic energy of a collection of gas particles is directly proportional to the kelvin temperature. The root mean square velocity increases with increasing temperature but decreases as the molar mass of a gas increases.
New Chemistry Video Playlist:
https://www.youtube.com/watch?v=bka20...
Access to Premium Videos:
https://www.patreon.com/MathScienceTutor
Facebook: https://www.facebook.com/MathScienceT...
Average Kinetic Energy of a Gas and Root Mean Square Velocity Practice Problems - Chemistry Gas Laws chemistry jobs | |
| 342 Likes | 342 Dislikes |
| 38,398 views views | 1.16M followers |
| Education | Upload TimePublished on 19 Sep 2017 |
Không có nhận xét nào:
Đăng nhận xét